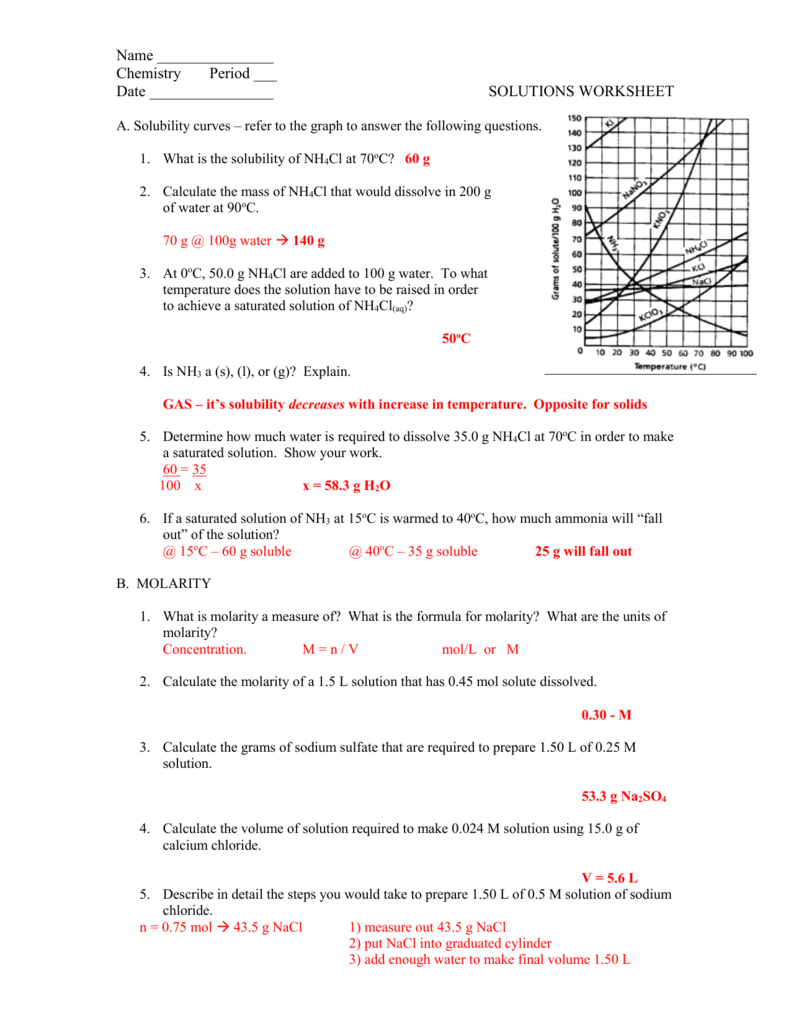
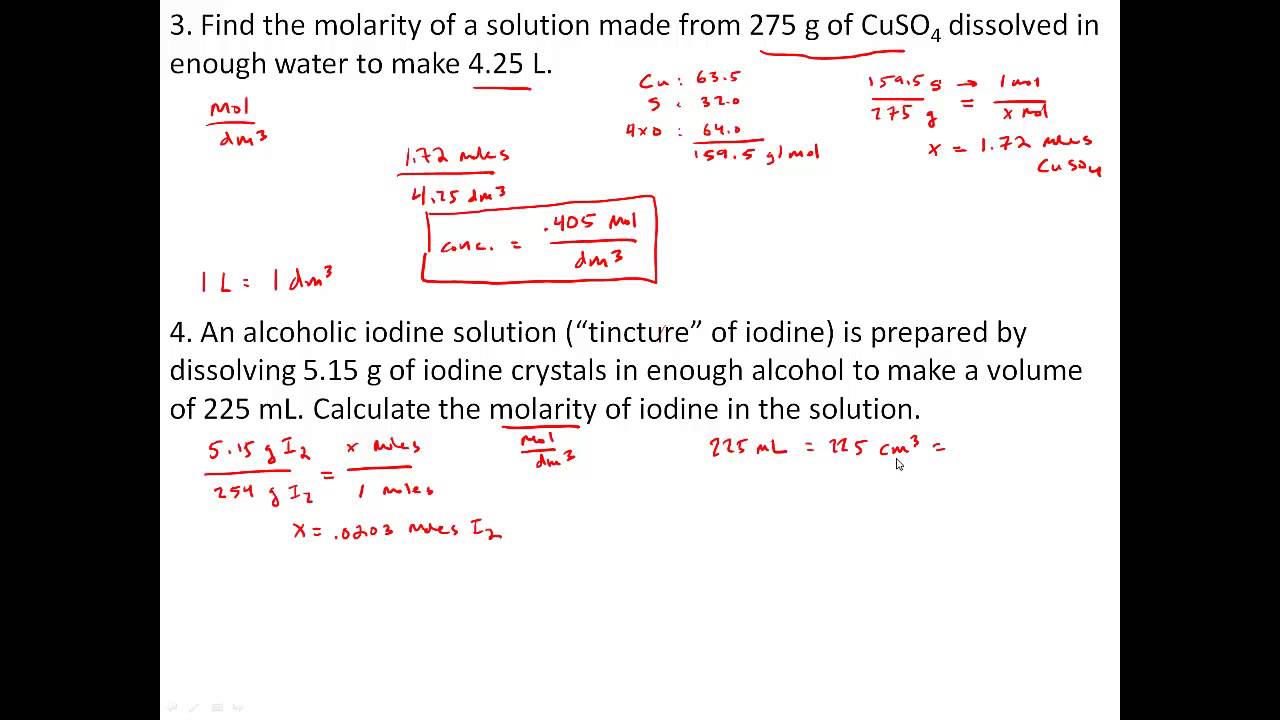
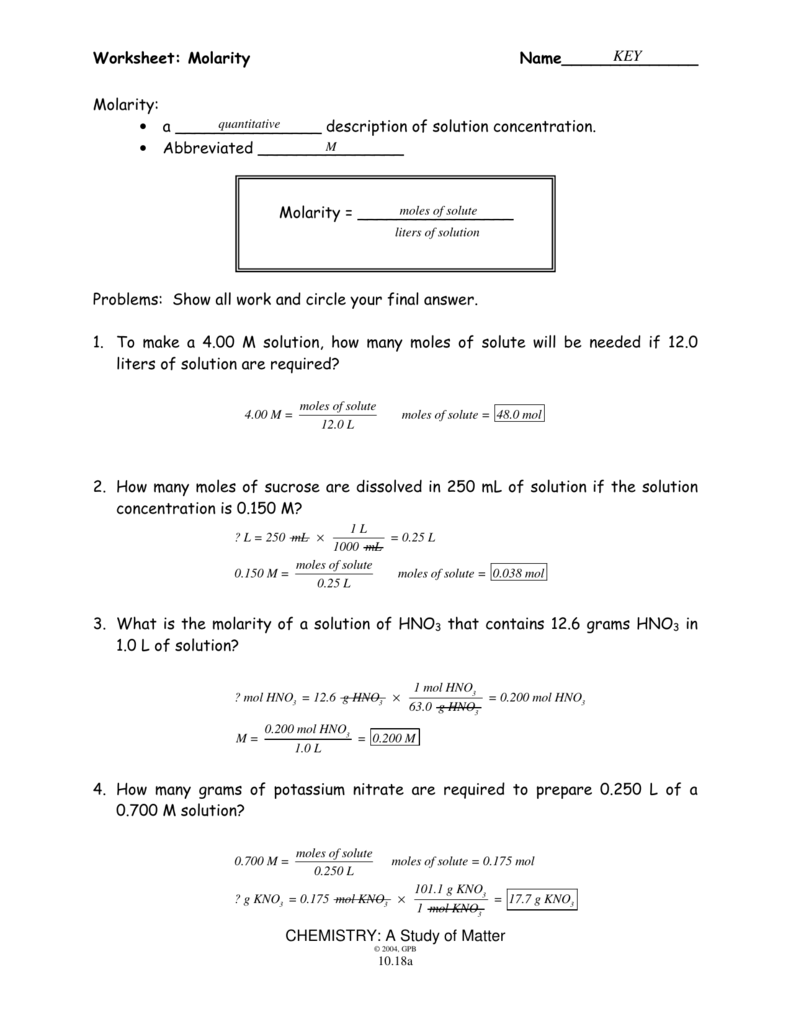
Worksheet Molarity Answers
Worksheet Molarity Answers - Molarity extra practice worksheet 1. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. 1) in this problem, simply solve using. What is the molarity of the following solutions given that:
Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. What is the molarity of the following solutions given that: Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Molarity extra practice worksheet 1. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) in this problem, simply solve using. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl?
Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); Molarity extra practice worksheet 1. 1) in this problem, simply solve using. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of the following solutions given that:
Chem 1020 Molarity worksheet, version 2 1. What is the molarity of a
Molarity extra practice worksheet 1. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following solutions given that: 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water?
Molarity Worksheet Answer Key
What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); 1) in this problem, simply solve using. Molarity extra practice worksheet 1. What is the molarity of the following solutions given that:
Molarity Practice Problems With Answers Molarity Problems Pr
What is the molarity of the following solutions given that: Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) in this problem, simply solve using. What is the molarity of 2.0 l of solution made from.
Molarity Practice Worksheet Answer New Molarity Practice Worksheet
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of the following solutions given that: What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Mw = 17.0305 g/mol m =.
10++ Molarity Calculations Worksheet Worksheets Decoomo
Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); What is the molarity of the following solutions given that: Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Molarity extra practice worksheet 1. Calculate molarity by dissolving 25.0g naoh.
Molarity
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) in this problem, simply solve using. Molarity extra practice worksheet 1. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of the following solutions given that:
Molarity Practice Worksheet
Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l.
Molarity Practice Worksheet Answer Elegant Molarity Practice Worksheet
Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Molarity extra practice worksheet 1. What is the molarity of the following solutions given.
Molarity Worksheet With Answers
Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. 1) in this problem, simply solve using. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following.
Molarity Problems Worksheet With Answers printable pdf download
Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. 1) in this problem, simply solve using. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. 1) 1.0 moles of potassium fluoride is dissolved.
Calculate Molarity By Dissolving 25.0G Naoh In 325 Ml Of Solution.
Mw = 17.0305 g/mol m = 10.5 m d= 0.9344 g/cm3 assume 1 l of 10.5 m nh3(aq); What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Molarity extra practice worksheet 1. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
1) In This Problem, Simply Solve Using.
What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following solutions given that: Molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml.