Worksheet Boyle S Law And Charles Law
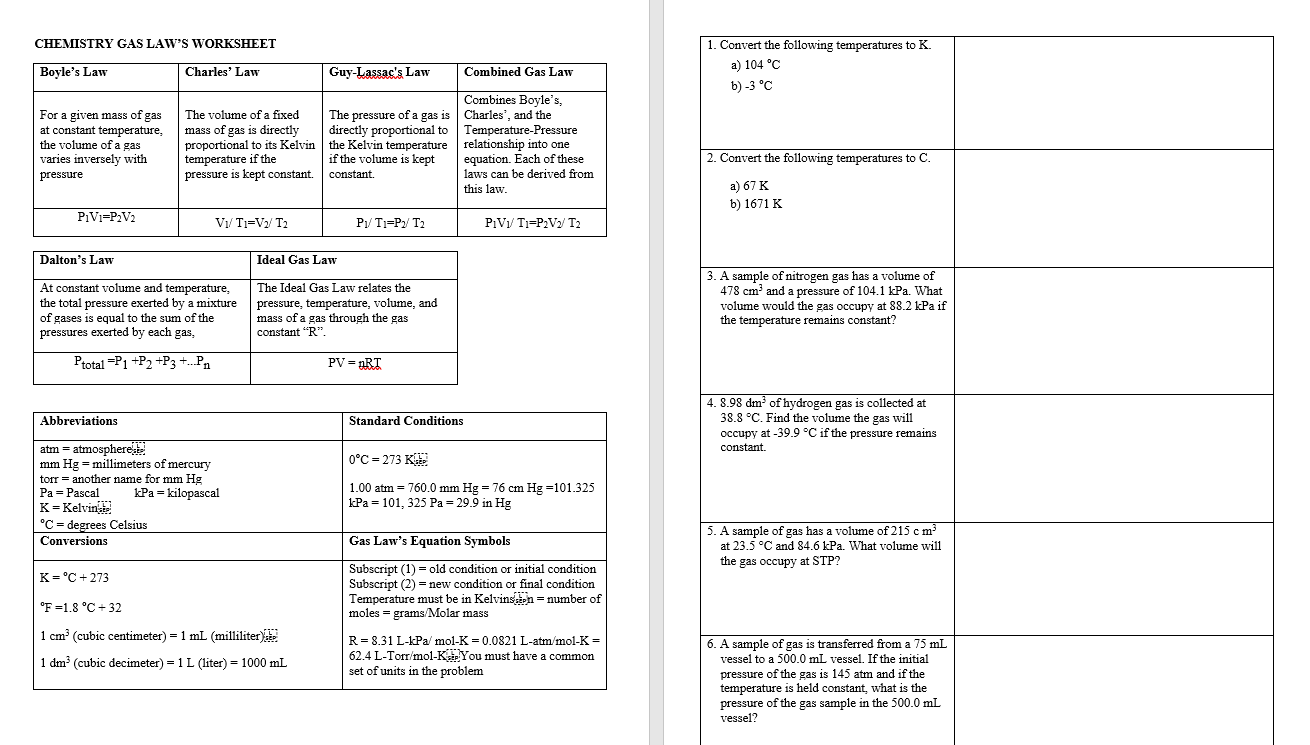
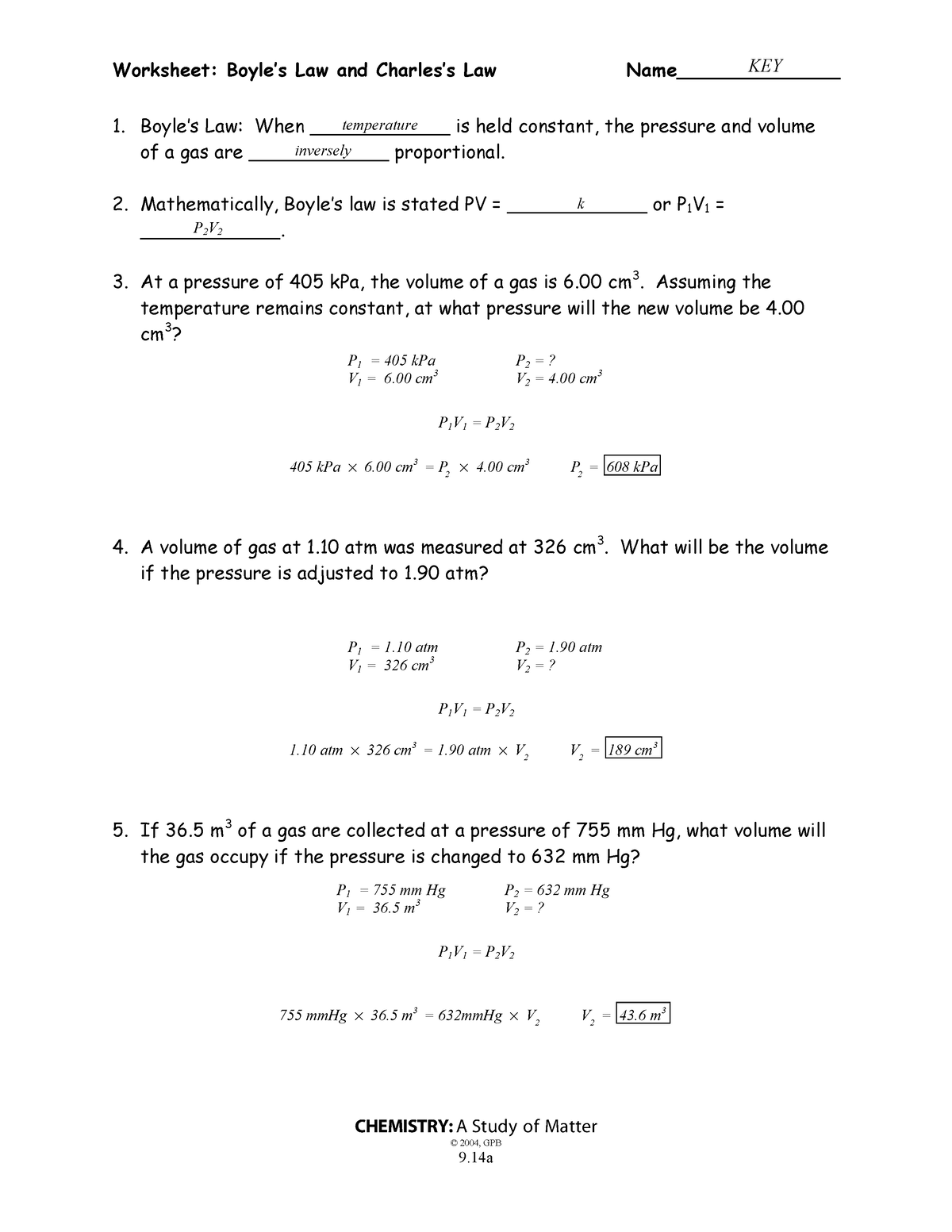
Worksheet Boyle S Law And Charles Law - A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. Complete each of the following showing all work and circling your final answer on all problems. To change a temperature expressed in degrees. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume?
When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the new volume? To change a temperature expressed in degrees. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. Complete each of the following showing all work and circling your final answer on all problems. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.
What is the new volume? Complete each of the following showing all work and circling your final answer on all problems. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. To change a temperature expressed in degrees. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the.
(DOC) Gas laws worksheet 2 boyles charles and combined (1)
Complete each of the following showing all work and circling your final answer on all problems. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. What is the new volume? To change a temperature expressed in degrees. When ____________ is held constant, the pressure and volume of a gas are ____________.
Solved CHEMISTRY GAS LAW'S WORKSHEET Boyle's Law Charles'
A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. To change a temperature expressed in degrees. What is the new volume? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. Complete each of the following showing all work and circling your final answer.
914a,b Boyles Law and Charless Law wkstKey Worksheet Boyle’s Law
A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What is the new volume? A gas with a volume.
Boyle's Law And Charles Law Worksheet Gas Laws
Complete each of the following showing all work and circling your final answer on all problems. To change a temperature expressed in degrees. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. What is the new volume? When ____________ temperature is held constant, the pressure and volume of a gas are.
Lesson Plan For Boyle's Law
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. To change a temperature expressed in degrees. A gas container is initially at 47 mm hg and.
Charles And Boyles Law Worksheet
A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. To change a temperature expressed in degrees. What is the new volume? When ____________ is held constant, the pressure and volume.
17 Charles And Boyle's Law Worksheet /
When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What is the new volume? A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. A gas with a volume.
Worksheet Boyle's Law And Charles Law Answers
Complete each of the following showing all work and circling your final answer on all problems. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the new volume? A gas with a volume of.
Worksheet – Boyle's Law, Charles's Law Worksheet Boyle’s Law
To change a temperature expressed in degrees. A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume? When ____________ temperature is held constant, the pressure and.
Boyle's Law and Charles's Law Worksheet for 10th 12th Grade Lesson
A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. To change a temperature expressed in degrees. What is the new volume? When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. When ____________ temperature is held constant, the pressure and volume of a gas are ____________.
What Is The New Volume?
A gas container is initially at 47 mm hg and 77 k (liquid nitrogen temperature.) what will the. To change a temperature expressed in degrees. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Complete each of the following showing all work and circling your final answer on all problems.
A Gas With A Volume Of 4.0L At A Pressure Of 205Kpa Is Allowed To Expand To A Volume Of 12.0L.
When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.