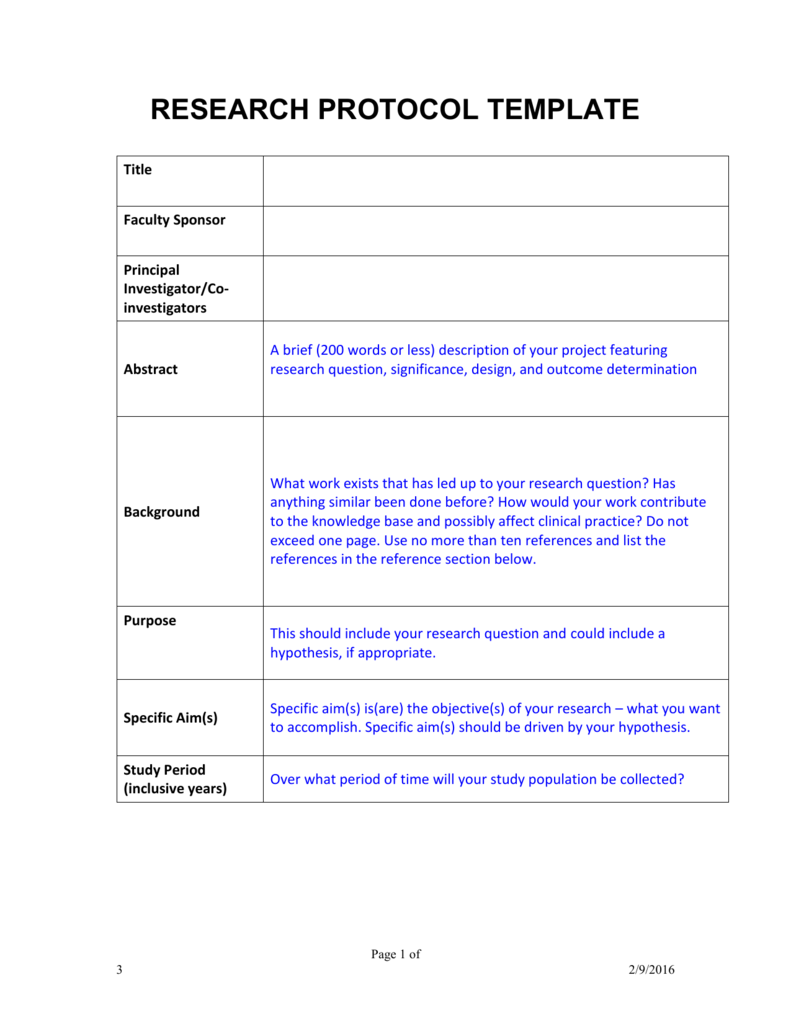
Research Protocol Template
Research Protocol Template - This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. This is the rsrb biomedical protocol template with required ur cabin language included in blue. The irb provides several protocol templates on this page. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: The natural history/observational protocol template, the.
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: The irb provides several protocol templates on this page. They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: This is the rsrb biomedical protocol template with required ur cabin language included in blue. The natural history/observational protocol template, the.
The natural history/observational protocol template, the. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: They follow the format of typical nih and industry multicenter protocols. The irb provides several protocol templates on this page. This is the rsrb biomedical protocol template with required ur cabin language included in blue. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated.
Sample Research Protocol Clinical Trial Risk
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated..
(PDF) Using a protocol template for case study planning
The irb provides several protocol templates on this page. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: The natural history/observational protocol template, the.
research protocol template
The irb provides several protocol templates on this page. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The natural history/observational protocol template, the. This is the rsrb biomedical protocol template with required ur cabin language included in blue. Nih applicants can use a template with instructional and.
Template Device protocol
This is the rsrb biomedical protocol template with required ur cabin language included in blue. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the. This protocol template is a tool.
Free Clinical Research Protocol Template Edit Online & Download
The natural history/observational protocol template, the. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. This is the rsrb biomedical protocol template with required ur cabin language included in blue. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the.
Quantitative Research Protocol Template
The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: They follow the format of typical nih and industry multicenter protocols. This is the rsrb biomedical protocol template with required ur cabin language included in blue. There are three templates to be.
Study Protocol Template
The natural history/observational protocol template, the. They follow the format of typical nih and industry multicenter protocols. This is the rsrb biomedical protocol template with required ur cabin language included in blue. There are three templates to be used for observational research: This protocol template is a tool to facilitate the development of a research study protocol specifically designed for.
Study Protocol Template
The irb provides several protocol templates on this page. They follow the format of typical nih and industry multicenter protocols. This is the rsrb biomedical protocol template with required ur cabin language included in blue. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical.
Minimal risk research protocol template in Word and Pdf formats page
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. They follow the format of typical nih and industry multicenter protocols. This is the rsrb biomedical protocol template with required ur cabin language included in blue. There are three templates to be used for observational research: Nih applicants can.
Minimal risk research protocol template in Word and Pdf formats page
There are three templates to be used for observational research: They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Nih applicants can use a template with instructional and sample text to.
This Protocol Template Is A Tool To Facilitate The Development Of A Research Study Protocol Specifically Designed For The Investigator Initiated.
They follow the format of typical nih and industry multicenter protocols. This is the rsrb biomedical protocol template with required ur cabin language included in blue. The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:
The Irb Provides Several Protocol Templates On This Page.
There are three templates to be used for observational research: