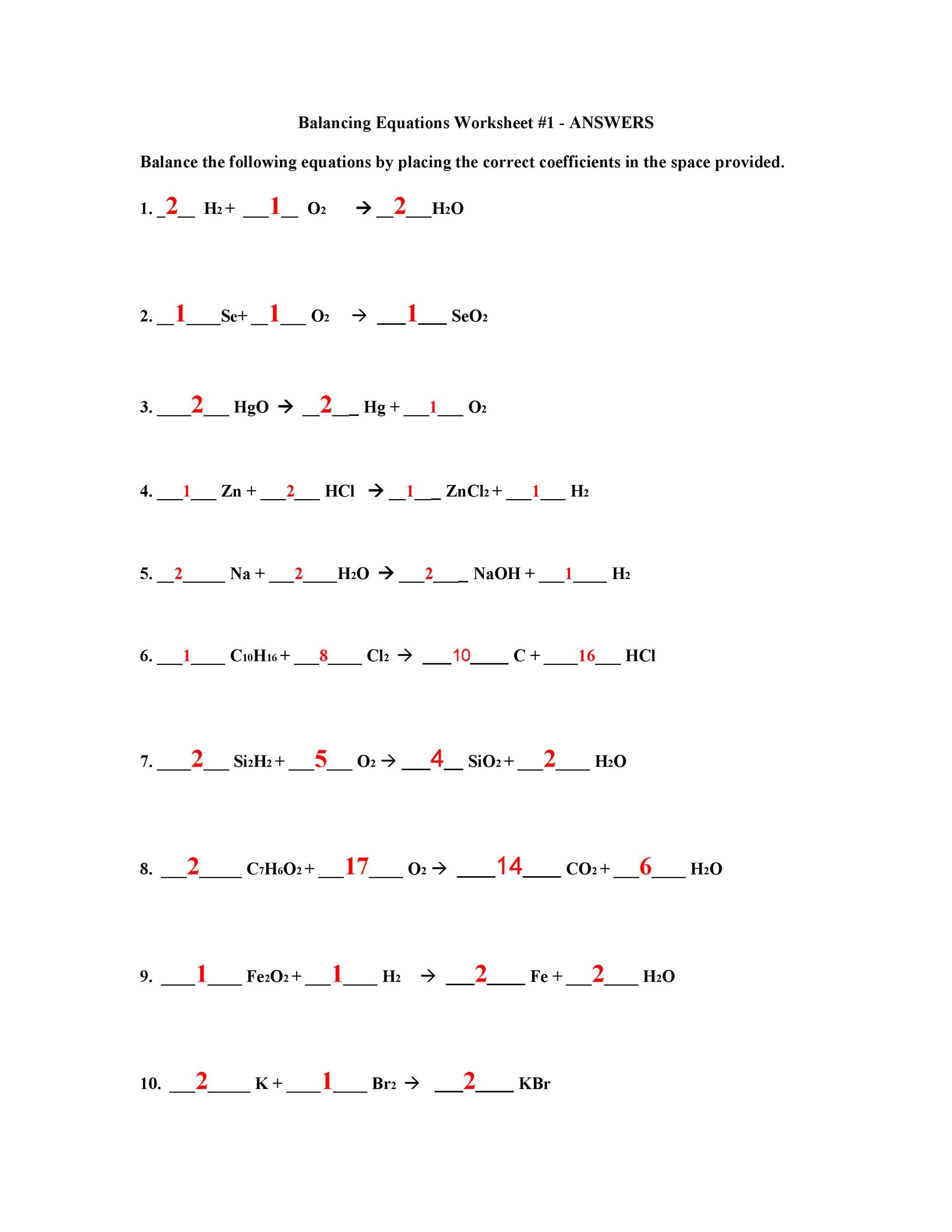
Balancing Chemical Equation Worksheet 1
Balancing Chemical Equation Worksheet 1 - 2mg + o 2 → 2mgo 5. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. Balance the following chemical equations. 2na + cl 2 → 2nacl 3. Write the word equations below as chemical equations and balance: 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. N 2 o 4 → 2no 2 4. 2h 2 + o 2 → 2h 2 o 2.
2mg + o 2 → 2mgo 5. 2na + cl 2 → 2nacl 3. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. 2h 2 + o 2 → 2h 2 o 2. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. Balance the following chemical equations. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. N 2 o 4 → 2no 2 4. Write the word equations below as chemical equations and balance:
2h 2 + o 2 → 2h 2 o 2. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. N 2 o 4 → 2no 2 4. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 2na + cl 2 → 2nacl 3. Balance the following chemical equations. 2mg + o 2 → 2mgo 5. Write the word equations below as chemical equations and balance:
Balancing Chemical Equations Worksheet 1 Science 9th Grade
Write the word equations below as chemical equations and balance: 2mg + o 2 → 2mgo 5. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 2na + cl 2 → 2nacl 3. 2h 2 + o 2 → 2h 2 o 2.
Balancing Chemical Equations Worksheet
1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. 2na + cl 2 → 2nacl 3. 2h 2 + o 2 → 2h 2 o 2. N 2 o 4 → 2no 2 4. 2mg + o 2 →.
Balancing Chemical Equation Worksheet
1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 2mg + o 2 → 2mgo 5. 2na + cl 2 → 2nacl.
Identifying And Balancing Chemical Equations Worksheets
Write the word equations below as chemical equations and balance: Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 2mg + o 2 → 2mgo 5. 2na + cl 2 → 2nacl 3. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl.
Chemistry Balancing Chemical Equations Worksheets
Write the word equations below as chemical equations and balance: 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 2na + cl 2 → 2nacl 3. Balance the following chemical equations. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2.
Worksheets For Balancing Chemical Equations
2mg + o 2 → 2mgo 5. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. 2na + cl 2 → 2nacl 3. 2h 2 + o 2 → 2h 2 o 2. N 2 o 4 → 2no.
Free Balancing Chemical Equations Worksheet [PDF] With Answers
2na + cl 2 → 2nacl 3. 2h 2 + o 2 → 2h 2 o 2. Balance the following chemical equations. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. Write the word equations below as chemical equations and balance:
49 Balancing Chemical Equations Worksheets [with Answers]
1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. 2h 2 + o 2 → 2h 2 o 2. Balance the following chemical equations. N 2 o 4 → 2no 2 4. 1) zinc and lead (ii) nitrate react.
Chemistry Balancing Chemical Equations Worksheet Worksheets Library
Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 2na + cl 2 → 2nacl 3. Balance the following chemical equations. Write the word equations below as chemical equations and balance: 2h 2 + o 2 → 2h 2 o 2.
Balanced Chemical Equation Worksheet Balancing Equations Che
2na + cl 2 → 2nacl 3. 2mg + o 2 → 2mgo 5. Balance the following chemical equations. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead.
Balancing A Chemical Equation Refers To Establishing The Mathematical Relationship Between The Quantity Of Reactants And Products.
Write the word equations below as chemical equations and balance: 2mg + o 2 → 2mgo 5. N 2 o 4 → 2no 2 4. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead.
2H 2 + O 2 → 2H 2 O 2.
2na + cl 2 → 2nacl 3. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2. Balance the following chemical equations.





![Free Balancing Chemical Equations Worksheet [PDF] With Answers](https://www.typecalendar.com/wp-content/uploads/2023/04/worksheet-on-balancing-chemical-equations.jpg)
![49 Balancing Chemical Equations Worksheets [with Answers]](https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-34.jpg)

